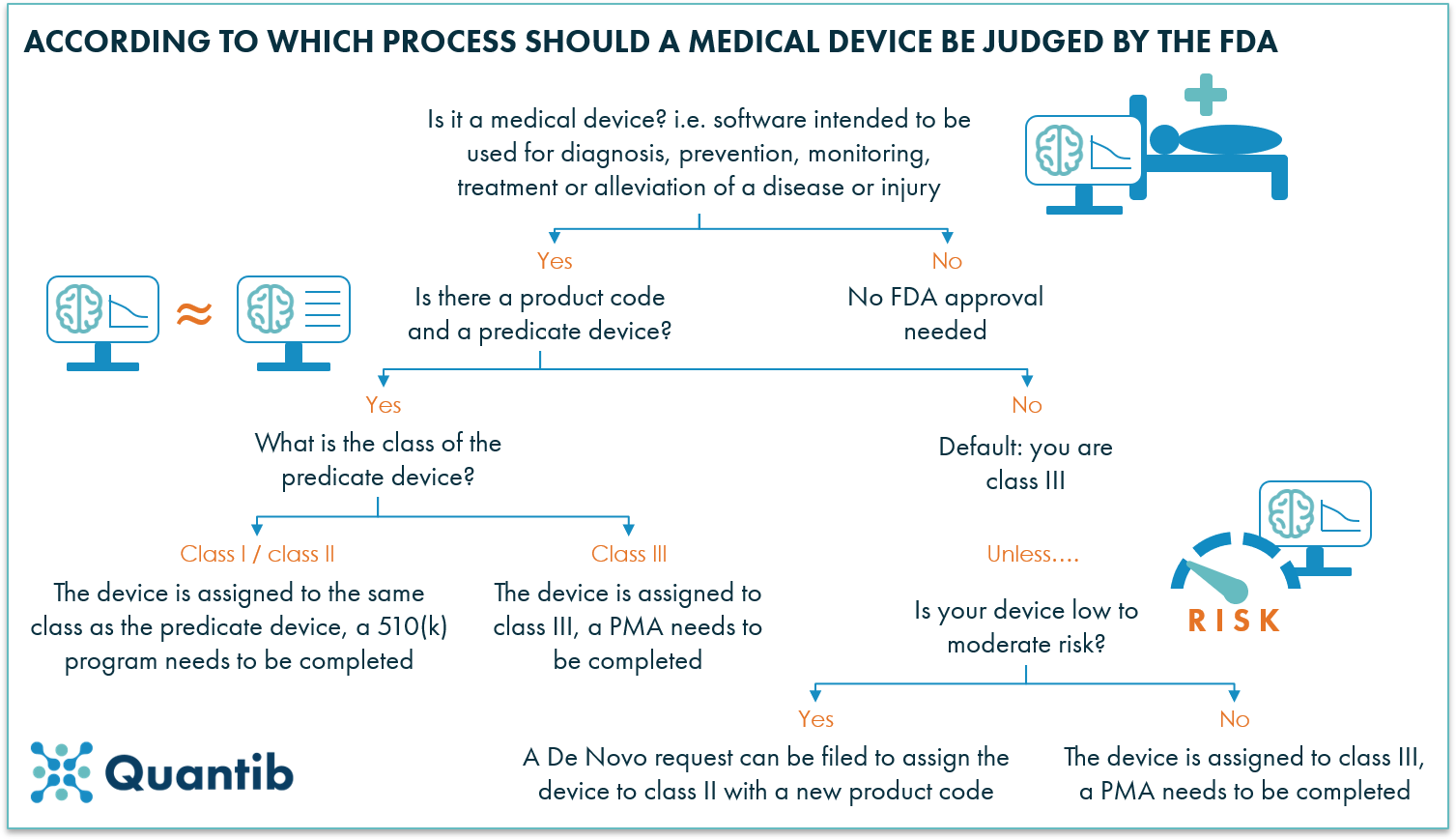
Software functions that are used in active patient monitoring to analyze patient specific medical device data and therefore are the focus of the fda s regulatory oversight including software.
Software as a medical device examples fda.
Standalone software that is used for diagnosis or treatment including software to evaluate the risk of trisomy 21 in a child based on serum markers in the mother.
Software as a medical device may be interfaced with other medical devices including hardware medical devices and other software as a medical device software as well as general purpose software.
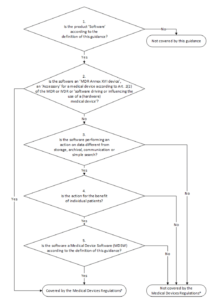
The mdcg gives examples of such medical device software mdsw.
The international medical device regulators forum imdrf of which the us fda is a member describes samd as software that may work on general purpose non medical computing platforms.
And may interface with other medical devices or other general purpose hardware and.
The term software as a medical device is defined by the international medical device regulators forum imdrf as software intended to be used for one or more medical purposes that perform these.